Experiment 2: Diffusion in liquids
This experiment was to show how diffusion worked, that particles move from a higher region of concentration to a lower region of concentration. I set up three glass bowls of water- in the first bowl, the water was cold. In the second bowl, the water was room temperature, and in the third bowl, the water was hot. I dropped a drop of blue food colouring into all three bowls and compared how fast the food colouring in the water diffused. In the first bowl, the food colouring diffused the slowest. This is because the cold temperature slowed down the speed of the randomly moving particles, hence causing the particles to diffuse the slowest. In the second bowl, the food colouring diffused at a moderate speed. The particles moved faster than the particles in the first bowl, but moved slower than the particles in the third bowl, hence its moderate speed. In the third bowl, the food colouring diffused the fastest. This is because the high temperature increased the speed of the randomly moving particles, hence causing the particles to diffuse the fastest.
Here are some photos in the experiment.
At the start of the experiment-
After some time in the experiment-
Thursday, February 3, 2011
Some experiments to help you understand better... (part 1)
Experiment 1: Osmosis- Potato slices in water
This experiment was to show how osmosis worked, that water particles move from a region of higher water concentration to a region of lower water concentration through a partially permeable membrane. I set up three glass bowls of water which were all at room temperature- the first bowl had no salt at all, the second bowl had one spoonful of salt, and the third bowl had two teaspoons of salt, and I put one potato strip measuring 3cm by 1cm by 1cm into each bowl. After 20 minutes, the potato strip in the first bowl measured 3.2cm by 1cm by 1cm, the potato strip in the second bowl still measured 3cm by 1cm by 1cm, and the potato strip in the third bowl measured 2.8cm by 1cm by 1cm. This is all a result of the process of osmosis. In the first bowl, there was a higher water potential outside the potato strip than inside the potato strip, hence the water in the bowl moved into the potato strip by osmosis, causing the potato strip to increase in length. In the second bowl, there was about the same number of molecules outside the potato strip and inside the potato strip, hence there was no noticeable difference in the length of the potato strip after the experiment. In the third bowl, there was a higher water potential inside the potato strip than outside the potato strip, hence the water from inside the potato strip moved out into the bowl by osmosis, causing the potato strip to decrease in length.
Here is a photo from the experiment:
This experiment was to show how osmosis worked, that water particles move from a region of higher water concentration to a region of lower water concentration through a partially permeable membrane. I set up three glass bowls of water which were all at room temperature- the first bowl had no salt at all, the second bowl had one spoonful of salt, and the third bowl had two teaspoons of salt, and I put one potato strip measuring 3cm by 1cm by 1cm into each bowl. After 20 minutes, the potato strip in the first bowl measured 3.2cm by 1cm by 1cm, the potato strip in the second bowl still measured 3cm by 1cm by 1cm, and the potato strip in the third bowl measured 2.8cm by 1cm by 1cm. This is all a result of the process of osmosis. In the first bowl, there was a higher water potential outside the potato strip than inside the potato strip, hence the water in the bowl moved into the potato strip by osmosis, causing the potato strip to increase in length. In the second bowl, there was about the same number of molecules outside the potato strip and inside the potato strip, hence there was no noticeable difference in the length of the potato strip after the experiment. In the third bowl, there was a higher water potential inside the potato strip than outside the potato strip, hence the water from inside the potato strip moved out into the bowl by osmosis, causing the potato strip to decrease in length.
Here is a photo from the experiment:
Tuesday, February 1, 2011
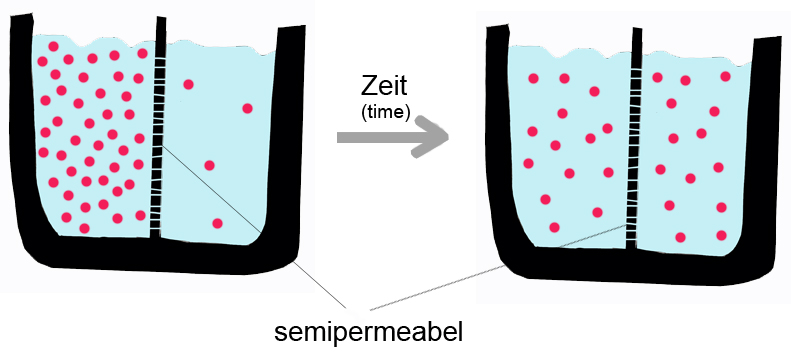
some diagrams and pictures about diffusion which would help you understand better
the above was taken from:
http://upload.wikimedia.org/wikipedia/commons/a/ac/Diffusion.jpg
 the above was taken from:
the above was taken from:
http://www.bio.miami.edu/~cmallery/150/memb/c8.7x11.diffusion.jpg
the above was taken from:
http://dickinsonn.ism-online.org/files/2009/11/626px-Scheme_simple_diffusion_in_cell_membrane-en_svg.png
http://upload.wikimedia.org/wikipedia/commons/a/ac/Diffusion.jpg

http://www.bio.miami.edu/~cmallery/150/memb/c8.7x11.diffusion.jpg
the above was taken from:
http://dickinsonn.ism-online.org/files/2009/11/626px-Scheme_simple_diffusion_in_cell_membrane-en_svg.png
Subscribe to:
Posts (Atom)